September 2006
Wildebeests of the Serengeti
![]()
Migrating in great numbers, the signature antelope of the African savanna must dodge
predators, drought, and human development. On the side, it shapes its own habitat.
![]()
By Richard D. Estes
|
But from the human perspective, the wildebeest may still need a champion; the lion, after all, is “king,” and even among the herbivores, the elephant and the giraffe cut more dramatic figures. And so, because the wildebeest has been the main focus of my field studies for four decades, I feel the duty and enjoy the privilege of representing the species to my own kind. For the past twenty-five years I have published the Gnusletter of the Antelope Specialist Group of the World Conservation Union. Had I been among the early people who hunted and gathered on the African savannas, I’m sure the wildebeest would have been my totem, and I would have drawn pictures of it on cave walls.
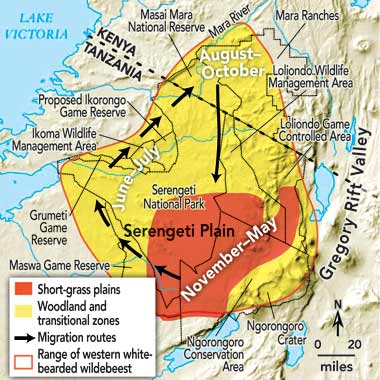
The term “wildebeest” actually refers to two species, Connochaetes taurinus and C. gnou, thought to have split from a common ancestor at least a million years ago. Biologists also recognize five distinct subspecies of C. taurinus. Of all the wildebeests, only the western white-bearded wildebeest, C. t. mearnsi, still thrives in the immense migratory herds that are the hallmark of wildebeest adaptation. An estimated 1.25 million animals range across Tanzania’s Serengeti National Park and adjacent regions, most migrating some 300 miles a year [see maps below].
|
Unfortunately, all the other major wildebeest populations have crashed. The chief reason is that the ever-expanding human population, with its demand for land for agriculture and domestic livestock, has been interfering with the wildebeests’ seasonal movements. In 1983, for instance, between 50,000 and 80,000 wildebeests perished when a fence blocked their escape from Botswana’s drought-stricken Central Kalahari Game Reserve [see “Good Fences, Good Neighbors?” by Graciela Flores, Natural History, June 2006].
Even the Serengeti herds have suffered calamities. In the late 1880s, the rinderpest virus was introduced into the continent by Indian cattle brought in for the Italian army, which was establishing a colonial presence in the Horn of Africa. Within a few years, the virus killed as many as 90 percent of the continent’s buffalo and cattle, as well as untold numbers of wildebeests and many other antelopes.
Yet the survivors of the pandemic, whose exposure earned them at least partial immunity, began rebuilding their populations within a decade or two. By 1963, rinderpest had disappeared from the Serengeti wildebeests. That population then multiplied fivefold, stabilizing at its present level of 1.25 million (give or take 10 percent) by the mid-1970s.
In the Serengeti, at least, it is still possible to observe the wildebeest’s exquisite adaptations, both physical and behavioral, to a complex landscape. For one thing, the wildebeest must contend with a habitat where access to food and water depends on timing and location that can vary drastically from year to year. For another, the wildebeest must share the landscape with other animals—both with other herbivores, including other migrants, as well as with predators. Because the population has bounced back so robustly, the animal’s future might seem assured.
| ||
Wildebeests are particularly well suited to harvest the abundant short grasses that cover certain semiarid plains during the rainy seasons. The best-known such habitat is provided by the plains of the eastern Serengeti, where an impermeable hardpan underlies a fertile layer of volcanic soil. The grasses there are highly nutritious because they take up calcium and phosphorus trapped by the hardpan. They produce abundant forage during the short rainy season of November through December and, more predictably, in the long rains of April and May. Meanwhile, the nutrients in the manure wildebeests spread across the grasslands are recycled by millions of dung beetles.
In good years the pastures remain green across the seasonal gap between the rains, from January through March. But from June through October there is little or no rain, and the green of the short-grass plains turns to tan. The animals then have little choice but to leave their pastures in search of grazing and water in adjoining regions—where there is more rainfall, and the grasses are taller (though less nutritious) and stay greener longer.
The wildebeest’s feeding apparatus—a wide muzzle, a broad row of incisors, and flexible lips—is well adapted for taking big bites of short grass. And in overall build the wildebeest has evolved to survive the rigors of migration and evade predators. The body mass is concentrated in the upper torso and thick neck, and is supported by long, relatively spindly legs, which enable the animal to run in bursts as fast as forty miles an hour. In addition, the wildebeest has high shoulders and a back that slopes down to lower hindquarters, a configuration that locomotion specialists believe is energetically efficient for traveling long distances at an easy canter. The spotted hyena, the ranking predator of wildebeests, has a similar conformation.
The openings of the wildebeest’s nostrils are equipped with flaps that help filter the dust stirred up by thousands of migrating animals. Pungent secretions from glands in the front hooves help wildebeests follow each other by scent, even on the darkest nights.
|
||
The protagonist of the Serengeti, the western white-bearded wildebeest, C. taurinus mearnsi, has the darkest coloration of the five recognized subspecies of C. taurinus. It also features the shortest horns and the most vociferous bulls. By weight, wildebeests are the sixth largest of Africa’s seventy-one antelope species, but C. t. mearnsi is the smallest subspecies: females average about 360 pounds, males about 460. Small size can be an advantage in hard times; in any event, it enables more individuals to concentrate on a pasture. Mitochondrial DNA studies by geneticist Nicholas J. Georgiadis, director of the Mpala Research Centre in Kenya, indicate that the subspecies has been separated for millennia from its closest relative, the eastern white-bearded wildebeest, by the western wall of the Gregory Rift Valley.
The wildebeest and two other major migratory species—the zebra and Thomson’s gazelle—return to the short-grass plains as soon as the rains begin anew, usually in November. Births among all three species peak there from December through March. Even at the height of the calving season, however, wildebeests keep on the move from pasture to pasture. That need has led wildebeests to abandon a strategy practically universal among other antelope species, as well as deer. The calves of most antelopes remain too feeble to outrun predators for days or even weeks after they are born. So the prevailing survival strategy among those species is for newborns to hide until they grow strong enough to flee.
|
In large part, wildebeests solved those problems by developing what are probably the most precocious calves of any ungulate. Newborns can gain their feet in as little as three minutes, and the average is only seven minutes. Furthermore, instead of seeking seclusion before and after calving, as do typical “hider” antelopes, pregnant wildebeests in migratory populations gather together on calving grounds and drop calves by the dozens between dawn and midday. Once the newborns have gained their feet and have suckled for the first time, they are led into the nearest nursery herd, an association of mothers and their nursing young. The presence of calves that are a few days older—and by then hard to catch—helps conceal the most vulnerable day-old calves.
Wildebeests also took another important evolutionary step for protecting their calves: about four-fifths of the yearly crop is born within just a few weeks. The concentrated calving season is comparable to that of caribou in the Arctic. The difference is that the abbreviated caribou calving period is an adaptation to the short Arctic summer, when newborn calves are most likely to survive. Among wildebeests, the principal environmental pressure that drives the calving pattern comes from predators: spotted hyenas, first and foremost, but also cheetahs, lions, and wild dogs.
|
||
Migration is another key factor in minimizing predation. After ranging the short-grass plains for six or seven months, the Serengeti wildebeests abandon them soon after the long rains end in May. The herds then spend the drier months mowing the tall grasses of the so-called woodland zone—which actually encompasses typical savannas (tree-studded grasslands) as well as open plains interspersed with woodlands of varying density. Here, too, armies numbering many thousands of wildebeests keep on the move. In contrast, hyenas and lions, the main predators, are tied to specific territories. They cannot simply follow the wildebeests wherever they go without trespassing on property defended by rival clans and prides.
How, I’ve long wondered, do the wildebeests of the Serengeti synchronize their reproduction? After all, the dramatic changes in the length of the day and night, which can serve as clues to the seasons in the temperate latitudes of southern Africa, are hardly noticeable in the tropics. More than forty years ago I hypothesized that the calling of the bulls triggered and synchronized estrus. The noise and confusion of the Serengeti rut, which coincides with the migration away from the short-grass plains and into the woodland zone, is a spectacle unequalled among land mammals. The sound and fury come from bulls staking claims to territories and competing to round up and mate with cows. Indeed, the bulls inseminate some 600,000 females in less than a month. How they manage to do it, though, is mystifying. All the action an observer is likely to catch goes into getting and holding females coveted by every neighboring bull.
My hypothesis about the source of synchronized estrus is being tested as part of a study of the reproductive physiology of Serengeti wildebeests. In 2003, a month before the rut, two groups of captured cows (one group penned with a bull) were continuously exposed to the recorded calls of rutting bulls, while a third, control group was kept isolated. The analysis of the thousands of samples of the captive cows’ dung, collected throughout the study, is still underway. If my hypothesis is correct, the levels of reproductive hormones in the dung will show that estrus was more closely synchronized in the cows exposed to rutting calls than it was in the control group.
But assuming I was right, what sets off the bulls? Maybe the fecal-hormone assays will show some changes in the females’ hormones that could arouse them. Or perhaps the males are simply reaching top condition at the end of the rains, attaining maximum testes size and testosterone production. Several months of increasing territorial activity precede the rut, and mating continues for as long as three months after the rut peaks. Indeed, some territorial activity is seen year round, which is good evidence that active bulls are ready and willing to breed. But one way or another, male aggression and sexuality reach fever pitch during the few weeks of the rutting peak.
Whatever stimulates the bulls, the dynamics of how they manage females and establish territories, both on the move and in more permanent locations, are complex. The prevailing system, in which large aggregations of
| ||
The rest of the Ngorongoro wildebeest population migrated between pastures affording the best grazing on the hundred-square-mile crater floor, in aggregations of several thousand animals. The aggregations included competing, mature bulls, as well as noncompeting bachelor males (ranging from yearlings to oldsters), females, and young. When such an aggregation settled on a pasture, the accompanying mature bulls began staking claims to territories, pushing noncompeting males out to peripheral—and often substandard—grazing grounds and rounding up groups of females and young. Temporarily, then, the aggregation would be fragmented much like a resident herd, until the time came to migrate in search of new pasture.
In a resident population, one bull controls, on average, a territory of perhaps one hectare (a bit bigger than two football fields). That is actually a remarkably small area for such a big animal (a hartebeest bull, in contrast, may control a territory a hundred times as large). When an aggregation of wildebeests happens to move into a resident neighborhood, however, the spacing may be effectively halved, as outsider bulls infiltrate and temporarily set up shop amid the resident bulls.
At the height of the annual Serengeti rut the density is even higher: the average increases to three competing bulls per hectare. In other words, as many as 300 bulls can be packed into one square kilometer, whereas a single hartebeest may defend a territory that size. And it is not unusual to find two or even three bulls under the same tree, moving through a crowd of a hundred cows that are seeking shade from the midday sun. At first glance it might seem they are tolerating each other’s close presence, willingly sharing the wealth of females. In fact, the packed bodies are just keeping them from catching sight of one another. The same screening effect enables noncompeting males to mingle in dense concentrations and thereby gain access to the best grazing, as long as they can escape the notice of territorial bulls.
The fact that males and females look so much alike promotes their association in mixed herds. The territorial males do stand out in the crowd, but that is primarily because of their behavior. Nevertheless, the physical similarities present a puzzle. According to Darwin’s theory of sexual selection, reproductive competition between males, in systems that enable the fittest males to monopolize matings, should lead to the development of conspicuous male secondary sex characteristics. For example, males endowed with larger size and weapons are most likely to prevail, and any such traits that are heritable will be passed along to their male descendants, leading to pronounced differences between males and females (so-called sexual dimorphism).
But that’s not what happens among wildebeests. After years of pondering the puzzle, I developed my own hypothesis, that natural selection suppresses development of conspicuous male secondary sex characteristics in species that regularly associate in mixed herds. To be sure, competition among males of the same age and stage of development (peer competition) promotes the evolution of such characteristics. But as soon as young males look different from females, breeding males begin to treat them as potential rivals. Sooner or later, young males subjected to such despotic competition are driven out of the female herd and the familiar home range, and hence exposed to greater danger. So the longer adolescent males look and act like females, the better their chances of postponing eviction and surviving to reproduce.
There is a hitch, however, to how male sexual development plays out in bovids, the large family of ungulates that comprises the antelopes and other hollow-horned ruminants, such as cattle, goats, and sheep. Horns are the essential weapons with which bovid males compete for dominance; horns are also the most basic male secondary sex characteristics. Unlike the bony antlers of deer, they are permanent structures. Peer competition promotes their growth as early as possible, presenting a dilemma for the young male. A factor that comes into play here is the females’ interest in protecting their male offspring from the aggression of breeding males. How can they help? One way is by growing horns themselves—and, among many bovids, including wildebeests, that is just what they do. Presto! Horns of similar shape and size carried by both sexes cease to be male sex symbols. As a consequence, horn development can (and does) begin much earlier than in species with hornless females.
In most species in which both sexes have horns, the females’ horns do not grow as large or robust as the males’ do. The reason, I presume, is that the females’ mimicry of male horn development proceeds only to the stage at which young males are ready to leave the female herds and assert themselves in competition with other males. But in species where natural selection favors the continued association of adults of both sexes within herds,
|
||
In spite of years of observation, biologists still have much to learn about wildebeest ecology. For example, one way adult bulls express their aggressiveness is to use their horns to thrash small trees of a certain size and “whippiness.” The activity, which I call “horning,” reminds me of a boxer working out on a punching bag. The behavior is easily overlooked, though, which may explain why Serengeti ecologists have not taken its impact into account. Yet the effects are plain to see: repeated horning debarks stems, breaks branches, and eventually kills the aboveground part of a tree. Such damage is commonly mistaken for elephant browsing, though on close inspection the differences are obvious.
In 1986 I had the chance to look for horning damage in several parks of South Africa and Namibia, where wildebeest populations had crashed after fencing prevented their migrations. The signs of horning there were scarce, and I noted that bushes and trees were invading formerly open grassland. In contrast, there was a marked opening up of the Serengeti woodlands in the 1970s and 1980s, as the wildebeest population, finally freed of rinderpest, increased apace. Together with my systematic observations of horning, the demographic data convince me that wildebeests, along with elephants and fire, have had profound environmental effects. That may seem astonishing, but when you consider that in the Serengeti a quarter-million competing bulls have been at it year after year, it seems less incredible. By adding tree-horning to the better-known effects of feeding, trampling, and depositing manure, it becomes clear that this antelope actively creates and helps maintain the kind of habitat that it needs.
At the same time, the areas available for grazing and access to water determine the size of the Serengeti wildebeest population. The equilibrium varies from year to year, depending on rainfall and on how much grass is produced. When there is not enough food, the weakest members of the population starve. In the absence of the occasional severe drought, most of the culling occurs late in the dry season, when the calves, whose nutritional needs for growth put them at particular risk, are most likely to lose condition and eventually succumb to parasites and disease. Those factors imply that despite its success, the western white-bearded wildebeest remains vulnerable.
Then there are the pressures of human development. The Serengeti wildebeests’ access to Lake Victoria, only a mile or so from the western boundary of Serengeti National Park, was cut off years ago by lakeside settlement. How long can Tanzanian politicians resist the demands of the burgeoning human population for further expansion? Other threats are building in Kenya, even in the Masai Mara National Reserve, where most of the Serengeti wildebeest population ends up during the dry season.
Fortunately, the Kenyan government has put an indefinite hold on a hydroelectric scheme to divert a major tributary of the Mara River. Still, the energy project is not dead. Even more serious is the threat posed by the destruction of the Mau Forest, the catchment for the Mara River and other rivers. Illegal invasion of the forest by settlers is continuing apace. Tree-cutting, clearing, and farming have already diminished the flow of the Mara. Soil erosion is muddying the water and interfering with subsistence fishing. According to a study by the World Wildlife Fund, phosphates and nitrates in the river have already reached environmentally harmful concentrations. All those unfortunate developments are taking place against a background of global climate change that is creating an increasingly arid climate in East Africa.
If wildebeests could speak for themselves, I have no doubt that they would decry these developments. Since the animals are unable to, only we can speak out for their interests—and convince our fellow human beings that their interests are ultimately our own.
 |
Copyright © Natural History Magazine, Inc., 2006