The Origins of Form
Ancient genes, recycled and repurposed, control embryonic development in organisms of striking diversity.
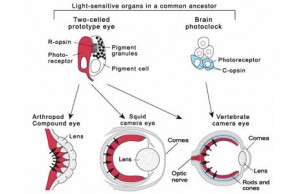
Formation of a vast range of eye types—the camera eyes of vertebrates and squids as well as the compound eyes of flies—is controlled by a single gene, called Pax-6. Recognizing the role of the Pax-6 gene has been a key piece of evidence in tracing the evolution of the eye. Each animal eye evolved from simpler photoreceptive structures in a distant common ancestor of the arthropods, cephalopods, and vertebrates. The ancestor possessed two kinds of light-sensitive organs (upper half of diagram), each one endowed with a distinct type of photoreceptor, as well as with light-sensitive proteins called R-opsin and C-opsin, respectively. One organ was a simple two-celled prototype eye; the other, called the brain photoclock, was a part of the animal’s brain and played a role in running the animal’s daily clock. The arthropod and squid retinas (red) incorporated the photoreceptor from the simple prototype eye, whereas the vertebrate eye incorporated both kinds of photoreceptor into its retina (red and blue). Rods and cones, the photoreceptors of human vision, are shown in blue. Their orientation with respect to the light source (black arrows) is opposite that of the photoreceptors in the arthropod and squid eyes.
Ernst Mayr once wrote:
If there is only one efficient solution for a certain functional demand, very different gene complexes will come up with the same solution, no matter how different the pathway by which it is achieved. The saying "Many roads lead to Rome," is as true in evolution as in daily affairs.
But Mayr’s view is incorrect. The architects of the modern synthesis expected the genomes of vastly different species to differ vastly. They had no idea that such different forms could be built with similar sets of genes. Stephen Jay Gould, in his monumental work, The Structure of Evolutionary Theory, saw the unexpected discovery of common body-building genes as overturning a major tenet of the modern synthesis.
There are not as many roads to Rome—or in other words, evolutionary paths to eyes and other complex structures—as biologists once thought. Natural selection has not repeatedly forged eyes from scratch. Rather, eye formation has common genetic ingredients, and a wide range of eye types incorporate parts, such as photoreceptor cells and light-sensing proteins, that have long been under the command of the Pax-6 gene.
Other tool-kit genes have been identified that take part in building various kinds of limbs, hearts, and other structures. Because parts of the genetic tool kit are shared among most branches of the animal kingdom, they must date back, at least, to some common ancestor of those branches. That would place their origin far back in time, before the Cambrian explosion that marked the emergence of large, complex animal bodies, more than 500 million years ago.
Here, then, is another somewhat counterintuitive insight from evo-devo: One might think that increases in animal complexity and diversity would be driven by the evolution of new genes. But it is now clear that most body-building genes were in place long before most kinds of animal body plans and complex organs emerged.
The discovery of such an ancient genetic tool kit, as exciting and rewarding as it is, raises a conundrum. If the sets of body-building genes among animals are so similar, how do such vast differences in forms arise?
Studies of many animal groups have shown that the diversity arises not so much from the content of the tool kit, but from how it is used. Various animal architectures are the products of applying the same genetic tools in different ways. For example, one of the most obvious features of large, complex animals such as vertebrates (fishes, amphibians, reptiles, birds, mammals) and arthropods (centipedes, spiders, crustaceans, insects) is their construction from repeating parts. Segments are the building blocks of arthropod bodies, vertebrae the building blocks of backbones. In both cases, important structures emerge from subsets of these building blocks—the many appendages of arthropods from their segments, the ribs of vertebrates from the vertebrae.
One of the dominant themes in the large-scale evolution of these animal bodies is change in the number and kind of repeating parts. The major features that distinguish classes of arthropods are the number of segments and the number and kind of appendages. Similarly, vertebrates differ fundamentally in the number and kind of vertebrae (cervical, thoracic, lumbar, sacral).
Extensive study of arthropod and vertebrate development has shown that those major features depend on a set of tool-kit genes called Hox genes. In general, Hox genes shape the number and appearance of repeated structures along the main body axes of both groups of animals. Individual Hox genes govern the identity of particular zones along that main body axis, and determine where various structures will form.